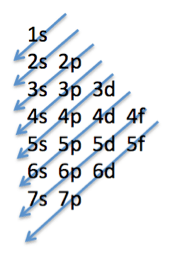
05.02 Electron Configuration
Quiz by Kelly Gallagher
Feel free to use or edit a copy
includes Teacher and Student dashboards
Measure skillsfrom any curriculum
Measure skills
from any curriculum
Tag the questions with any skills you have. Your dashboard will track each student's mastery of each skill.
With a free account, teachers can
- edit the questions
- save a copy for later
- start a class game
- automatically assign follow-up activities based on students’ scores
- assign as homework
- share a link with colleagues
- print as a bubble sheet
7 questions
Show answers
- Q1Electron configuration refers to the _____________ of electrons.arrangement in orbitalsspinmasscharge30s
- Q2Which is not a rule to follow when writing an atom's electron configuration?pauliconservation of massaufbauhund30s
- Q3The __________ states that electrons must occupy the lowest energy orbitals first.hund's rulePauli exclusion principleaufbau principlelaw of conservation of mass30s
- Q4The _________ states that an orbital may contain at most 2 electrons.Pauli exclusion principlehund's ruleHeisenberg Uncertainty Principleaufbau principle30s
- Q5According to ___________ electrons will maximize the number of electrons with the same spin before doubling up in an orbital.Pauli exclusion principleWolff's lawHund's ruleaufbau principle30s
- Q6____________ states that you can't know with certainty both where an electron is and where it's going next.Pauli exclusion principleHund's RuleThe Heisenberg Uncertainty PrincipleAufbau principle30s
- Q7__________ is a mathematical model describing the probability of finding an electron at a particular location in an atom.Hund's ruleatomic orbitalsPauli Exclusion PrincipleHeisenberg Uncertainty Principle30s