08.02 Covalent Bonds
Quiz by Kelly Gallagher
Feel free to use or edit a copy
includes Teacher and Student dashboards
Measure skillsfrom any curriculum
Measure skills
from any curriculum
Tag the questions with any skills you have. Your dashboard will track each student's mastery of each skill.
With a free account, teachers can
- edit the questions
- save a copy for later
- start a class game
- automatically assign follow-up activities based on students’ scores
- assign as homework
- share a link with colleagues
- print as a bubble sheet
12 questions
Show answers
- Q1Atoms gain ___________ when they share electrons and form bonds.protonsstabilitymassa charge30s
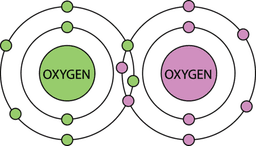
- Q2Covalent bonds are formed between ___________ & ___________.metals & non-metalsnon-metals & non-metalsmetals & metals30s
- Q3Atoms in covalent bonds ___________ electrons.donateshareexchangeaccept30s
- Q4When atoms bond covalently, they become ______________.an atoma moleculean alloya salt30s
- Q5Which of these is not a diatomic molecule?SNHO30s
- Q6When one pair of electrons is shared, a ___________ covalent bond is formed.singleionicdoubletransduction30s
- Q7When two pairs of electrons are shared, a __________ bond is formed.alloyionicdouble covalentquadruple covalent30s
- Q8When three pairs of electrons are shared, a ________ bond is formed.immediatedouble covalentdiatomictriple covalent30s
- Q9_________ covalent bonds are the strongest, and hardest to break apart.coordinatesingletripledouble30s
- Q10___________ covalent bonds result when one atom donates all of the shared electrons.singledoubleCoordinateionic30s
- Q11____________ explains whey there may be more than one way to draw electron structures of molecules.noble gas configurationdissociation energyactivation energyresonance30s
- Q12___________ is the energy required to break a bond between covalently bonded atoms.covalent chargeactivation energybond frequencydissociation energy30s