Atomic structure and Isotopes
Quiz by Gomez, Gabriela E
Feel free to use or edit a copy
includes Teacher and Student dashboards
Measure skillsfrom any curriculum
Measure skills
from any curriculum
Tag the questions with any skills you have. Your dashboard will track each student's mastery of each skill.
With a free account, teachers can
- edit the questions
- save a copy for later
- start a class game
- automatically assign follow-up activities based on students’ scores
- assign as homework
- share a link with colleagues
- print as a bubble sheet
10 questions
Show answers
- Q1Atoms of the same element with a different number of neutrons are ____________.quarksionsprotonsisotopes45s
- Q2Which subatomic particle(s) are found in the nucleus of an atom?protons onlyprotons and electronsneutrons and electronsprotons and neutrons45s
- Q3Which subatomic particle has a similar mass to a proton?electronsneutronsphotonnucleus60s
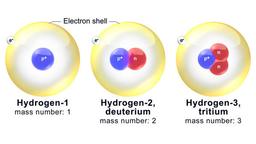
- Q4What do all of these isotopes of hydrogen have in common?Same number of protonsThey don't have anything in commonSame atomic massSame number of neutrons60s
- Q5What part of the atom is labeled in the diagram?neutronsNucleuselectronsOrbital45s
- Q6What is the atomic number for an element with three protons and six neutrons?63960s
- Q7What is the chemical symbol for an element with 3 protons and 6 neutrons?OxygenFluorineCarbonLithium60s
- Q8What is the atomic mass of Fluorine?172091960s
- Q9Which of the following pairs are isotopes of the same element?Helium-4 and Hydrogen-2Sulfur-32 and Oxygen-16Carbon-14 and Carbon-1360s
- Q10True or False. In an isotope the only thing that changes is the number of protons in an atom.TrueFalse45s