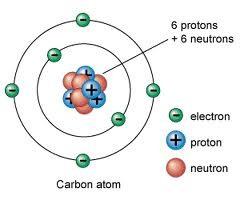
Atomic Structure- G9 ONLY
Quiz by Christina BOYLE
Feel free to use or edit a copy
includes Teacher and Student dashboards
Measure skillsfrom any curriculum
Measure skills
from any curriculum
Tag the questions with any skills you have. Your dashboard will track each student's mastery of each skill.
With a free account, teachers can
- edit the questions
- save a copy for later
- start a class game
- automatically assign follow-up activities based on students’ scores
- assign as homework
- share a link with colleagues
- print as a bubble sheet
12 questions
Show answers
- Q1smallest unit of matteratom30s
- Q2the center of the atom that contains neutrons and protons; where most of the mass of an atom is locatednucleus30s
- Q3the outer portion of an atom that contains electrons; where most of the volume of an atom is locatedelectron cloud30s
- Q4neutrally charged subatomic particles with a mass of 1 amu; located in the nucleusneutron30s
- Q5positively charged subatomic particles with a mass of 1 amu; located in the nucleusproton30s
- Q6the average mass of all atoms of an elementatomic mass30s
- Q7the rounded mass of an atom; protons + neutronsmass number30s
- Q8the number of protons in an atom; the number that identifies an elementatomic number30s
- Q9describes the ratio of the atoms of a particular substance (water = H2O)chemical formula30s
- Q10A subatomic particle that has a negative chargeElectron30s
- Q11the chart scientists use to organize and classify all the known elementsPeriodic Table of Elements30s
- Q12One or two letters that represent element.element symbol30s