Atomic Theory Exam Review Questions
Quiz by Darrien James
Feel free to use or edit a copy
includes Teacher and Student dashboards
Measure skillsfrom any curriculum
Tag the questions with any skills you have. Your dashboard will track each student's mastery of each skill.
- edit the questions
- save a copy for later
- start a class game
- automatically assign follow-up activities based on students’ scores
- assign as homework
- share a link with colleagues
- print as a bubble sheet
- Q1
What is the electron configuration of Calcium?
1s22s22p63s23p64s1
1s22s22p63s23p5
1s22s22p63s23p64s2
1s22s22p63s23p6
30s - Q2
What is the electron configuration of N3- ?
1s22s22p4
1s22s22p5
1s22s22p63s23p6
1s22s22p6
30s - Q3
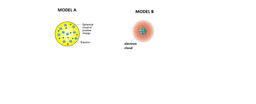
Which of the following models would represent very reactive metals?
Model 3 only
Model 2 and 3
Model 2 only
Model 1 and 2
30s - Q4
Which of the following is the most abundant isotope of carbon: 12C,13C, 14C? Explain.
13C is the most abundant isotope of carbon because its mass number is closest to the average atomic mass of carbon.
13C is the most abundant isotope of carbon because its atomic number is closest to the average atomic mass of carbon.
12C is the most abundant isotope of carbon because its mass number is closest to the average atomic mass of carbon.
12C is the most abundant isotope of carbon because its atomic number is closest to the average atomic mass of carbon.
30s - Q5
Four students were provided with Gallium's isotopic data in the following table (view image):
The students were asked to calculate the average atomic mass of Gallium. Which option represents the correct student's calculation?
(60.11/100)*(68.9256) + (39.89/100)*(70.9247)
(60.11*100)/(68.9256) + (39.89*100)/(70.9247)
(60.11)(68.9256) + (39.89)(70.9247)
(60.11)+(68.9256) +(39.89)+(70.9247)
30s - Q6
How many protons, neutrons and electrons are there in the following phosphorus anion?
15 protons, 16 neutrons, 18 electrons
15 protons, 16 neutrons, 15 electrons
16 protons, 15 neutrons, 18 electrons
15 protons, 15 neutrons, 18 electrons
30s - Q7
How many protons, neutrons and electrons are there in the following lead cation?
82 protons, 124 neutrons, 86 electrons
124 protons, 82 neutrons, 78 electrons
82 protons, 82 neutrons, 78 electrons
82 protons, 124 neutrons, 78 electrons
30s - Q8
When is light emitted during a flame test?
Light is emitted when the proton returns to the ground state from the excited.
Light is emitted when the atom is heated and the energy is used to move the electron to the excited state.
Light is emitted when the atom is heated and the energy is used to move the neutron to the excited state.
Light is emitted when the electron returns to the ground state from the excited.
30s - Q9
Examine the attached image, which of the following is supporting evidence that a new element is produced?
The newly produced atom has a different number of neutrons.
The newly produced atom has a different number of electrons.
All 3 atoms have the same number of protons
The newly produced atom has a different number of protons.
30s - Q10
Which of the following is the electron configuration of a non reactive element?
1s22s22p63s23p6
1s22s22p63s23p64s2
1s22s22p63s23p64s1
1s22s22p63s23p5
30s