Elements, compounds and mixtures
Quiz by Joelie Marcial
Feel free to use or edit a copy
includes Teacher and Student dashboards
Measure skillsfrom any curriculum
Measure skills
from any curriculum
Tag the questions with any skills you have. Your dashboard will track each student's mastery of each skill.
With a free account, teachers can
- edit the questions
- save a copy for later
- start a class game
- automatically assign follow-up activities based on students’ scores
- assign as homework
- share a link with colleagues
- print as a bubble sheet
20 questions
Show answers
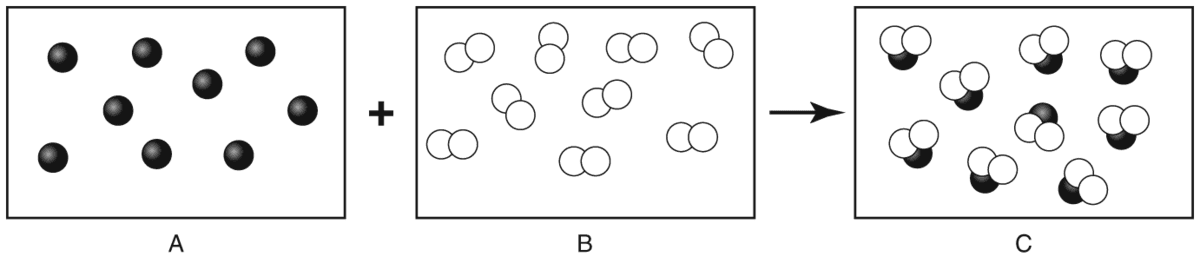
- Q1Reactant A and reactant B undergo a chemical reaction to form product C. What is reactant B?compounda nucleusan elementmixture60s
- Q2The diagram below shows what happens during the formation of a compound. The diagram shows two substances joining together to form a third substance. Which one is a compound?CAboth B and CB60s
- Q3The four items shown below were served at a dinner at Kim’s house. Each of the items is a mixture. Which one is a solution?GelatinApple JuiceItalian salad dressingwhipped cream60s
- Q4What type of substance is always made up of a single type of atom?compoundmixturemoleculeelement60s
- Q5Assume water and other substances are mixed to form a solution, a suspension, and a colloid. Which type of mixture contains the largest particles?solutioncolloidAll particles are the same size.suspension60s
- Q6Muddy water is an example of what type of mixture?colloidsolutionhomogeneoussuspension60s
- Q7What is true of all mixtures?All mixtures are made up of compounds.All mixtures are made up of elements.All mixtures are made up of atoms.All mixtures are made up of colloids60s
- Q8What kind of a heterogeneous mixture is the represented in the picture?solutionsuspensioncolloidelement60s
- Q9The four items below were part of a dinner. Each item is a mixture. Which of these mixtures is a suspension?DACB60s
- Q10How could you break down the compound calcium carbonate into the elements that make it up?by meltingwith a filterwith chemical changesby crushing60s
- Q11A glass of Kool-Aid is an example of asolutionsuspensioncompoundheterogeneous mixture60s
- Q12Which of these common substances is a homogeneous mixture?pizzasugar waterpure watertable salt60s
- Q13Solution is another name for what type of matter?heterogeneous mixturehomogeneous mixtureelementcompound60s
- Q14A substance contains an arrangement of different types of atoms joined together by chemical bonds. Which of the following classes of substances could this describe?all matterall elementsall compoundsall pure substances60s
- Q15Which of these substances is an example of a solution?milkmayonnaiseairgold60s