Intro to Chemistry - Vocabulary
Quiz by Jennifer Pendleton
Feel free to use or edit a copy
includes Teacher and Student dashboards
Measure skillsfrom any curriculum
Tag the questions with any skills you have. Your dashboard will track each student's mastery of each skill.
- edit the questions
- save a copy for later
- start a class game
- automatically assign follow-up activities based on students’ scores
- assign as homework
- share a link with colleagues
- print as a bubble sheet
- Q1
The study of the properties of matter and how matter changes.
chemistry
physics
chemicals
atoms
30s - Q2
Chemistry
The study of the properties of matter and how matter changes.
The study of the ability to exert a force causing displacement of an object.
The study of the energy transferred to or from an object via the application of force along a displacement.
The study of the physical makeup of the Earth and its atmosphere.
30s - Q3
A complex arrangement of negatively charged electrons arranged in defined shells about a positively charged nucleus
atom
compound
matter
subatomic particle
30s - Q4
Atom
A substance made from two or more different elements that have been chemically joined.
A particle that composes an atom.
Anything that has mass and takes up space.
A complex arrangement of negatively charged electrons arranged in defined shells about a positively charged nucleus.
30s - Q5
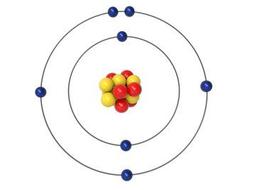
A structural model of an atom that shows the atom as a central nucleus containing protons and neutrons with the electrons in circular orbitals at specific distances from the nucleus.
Bear Model
Bohr Model
Rutherford Model
Boring Model
30s - Q6
Bohr Model
Model of an atom describing a sphere of positively charged matter in which electrons are embedded.
Model of an atom stating that atoms are indivisible and indestructible.
Model of an atom describing an atom to be a ball-like structure.
Model of an atom describing a small nucleus (positively charged) surrounded by negative electrons moving around the nucleus in orbit.
30s - Q7
Proton
A positively charged subatomic particle found outside the nucleus of every atom.
A neutrally charged subatomic particle found in the nucleus of every atom.
A positively charged subatomic particle found in the nucleus of every atom.
A negatively charged subatomic particle found in the nucleus of every atom.
30s - Q8
A positively charged subatomic particle found in the nucleus of every atom.
neutron
proton
element
electron
30s - Q9
Neutron
A negatively charged subatomic particle found in the nucleus of every atom.
A positively charged subatomic particle found in the nucleus of every atom.
A neutrally charged subatomic particle found in the nucleus of every atom.
A neutrally charged subatomic particle found outside the nucleus of every atom.
30s - Q10
A neutrally charged subatomic particle found in the nucleus of every atom.
neutron
electron
element
proton
30s - Q11
Electron
A positively charged subatomic particle found outside the nucleus of every atom.
A negatively charged subatomic particle found in the nucleus of every atom.
A neutrally charged subatomic particle found in the nucleus of every atom.
A positively charged subatomic particle found in the nucleus of every atom.
A negatively charged subatomic particle found outside the nucleus of every atom.
30s - Q12
A negatively charged subatomic particle found outside the nucleus of every atom.
element
neutron
electron
proton
30s - Q13
A regions surrounding the atomic nucleus containing a specific number of electrons.
electron shell
nucleus
atom
atomic number
30s - Q14
Electron Shell
A particle of matter that uniquely defines a chemical element.
The outer most layer of an atom that protects the nucleus.
A region surrounding the atomic nucleus containing a specific number of electrons.
A negatively charged subatomic particle that together with protons and neutrons form an atom's nucleus.
30s - Q15
Anything that has mass and takes up space.
electron shell
subatomic particles
proton
matter
30s