Kinetics )Arrhenius &mechanisms +vreyna
Quiz by Valerie Reyna
Feel free to use or edit a copy
includes Teacher and Student dashboards
Measure skillsfrom any curriculum
Tag the questions with any skills you have. Your dashboard will track each student's mastery of each skill.
- edit the questions
- save a copy for later
- start a class game
- automatically assign follow-up activities based on students’ scores
- assign as homework
- share a link with colleagues
- print as a bubble sheet
- Q1120s
- Q2120s
- Q3120s
- Q4120s
- Q5120s
- Q6300s
- Q7300s
- Q8300s
- Q9300s
- Q10
The frequency factor A in the Arrhenius equation, is associated with which of these aspects of collision theory?
the fraction of particles that collide with sufficient energy
the fraction of particles that have the correct orientation for a successful collision
the number of collisions per unit time
all of these
120s - Q11
Which of these is true regarding an SN1 reaction between (CH3)3CBr + OH- ----> (CH3)3COH + Br- ?
the rate law is rate = k[(CH3)3CBr][OH-]
the halogenoalkane is a primary one
increasing the concentration of hydroxide ion doubles the rate
the rate determining step is unimolecular
120s - Q12
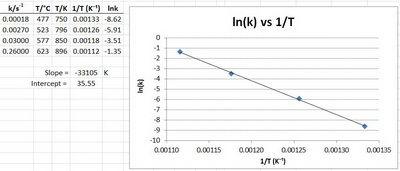
At 550oC, the rate constant for a reaction is 1.1 L mol-1s-1, and at 625oC the rate constant is 6.4 L mol-1s-1 ; what is the activation energy for this reaction?
140 KJ/mol
1.4 x 105 KJ/mol
6.7 x 104 J/mol
67 KJ/ mol
300s