Mid Check- 6.6A: Metals, Nonmetals, & Metalloids
Quiz by Masen Huddleston
Feel free to use or edit a copy
includes Teacher and Student dashboards
Measures 1 skill from
Track each student's skills and progress in your Mastery dashboards
- edit the questions
- save a copy for later
- start a class game
- automatically assign follow-up activities based on students’ scores
- assign as homework
- share a link with colleagues
- print as a bubble sheet
- Q1
Which of the following is a property of a non-metal?
conducts electricity and heats well
Is brittle and breaks easily
can be hammered into thin sheets
has a shiny, metallic luster
300s6.6a - Q2
All of the following are properties used to classify elements as metals, non-metals, and metalloids EXCEPT —
Malleability
Texture
Conductivity
Luster
300s - Q3
Iron (Fe) is classified as a metal while Silicon (Si) is classified as a metalloid. Which physical property do these two elements most likely have in common? They both —
exist as gases at room temperature
have a metallic shine or luster
are brittle and shatter easily
conduct electricity very well
300s - Q4
A student is given a sample of an unknown substance. He is asked to determine if it is classified as a metal, a metalloid, or a nonmetal. He discovered that the unknown element conducted some heat and electricity, had a shiny luster, and broke easily. This element is most likely a
cannot be determined
metalloid
nonmetal
metal
300s - Q5
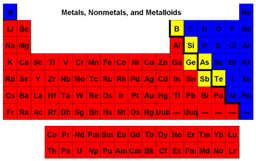
These items are on the right side of the Periodic Table (blue).
metalloids
nonmetals
metals
300s - Q6
Elements on either side of the dark zig zig line are called... (yellow)
metals
nonmetals
metalloids
300s - Q7
Most elements on the periodic table are...
nonmetals
metals
metalloids
300s - Q8
Which of the elements listed below would be considered a metalloid?
Sulfur
Neon
Silicon
Manganese
300s - Q9
Which table below correctly lists the properties of metals and nonmetals?
300s - Q10
A student tested the conductivity of four elements using an incomplete electrical circuit. When the element is placed in the circuit, the bulb lights up brightly, lights up dimly, or does not light up at all based on the element’s ability to conduct electricity. The table below shows the results of her investigation. Based on the results of the conductivity test, which element is classified as a metal?
Krypton
Copper
Arsenic
Hydrogen
300s