MIDTERM PRACTICE 1
Quiz by Oksana Tariche
Feel free to use or edit a copy
includes Teacher and Student dashboards
Measure skillsfrom any curriculum
Measure skills
from any curriculum
Tag the questions with any skills you have. Your dashboard will track each student's mastery of each skill.
With a free account, teachers can
- edit the questions
- save a copy for later
- start a class game
- automatically assign follow-up activities based on students’ scores
- assign as homework
- share a link with colleagues
- print as a bubble sheet
24 questions
Show answers
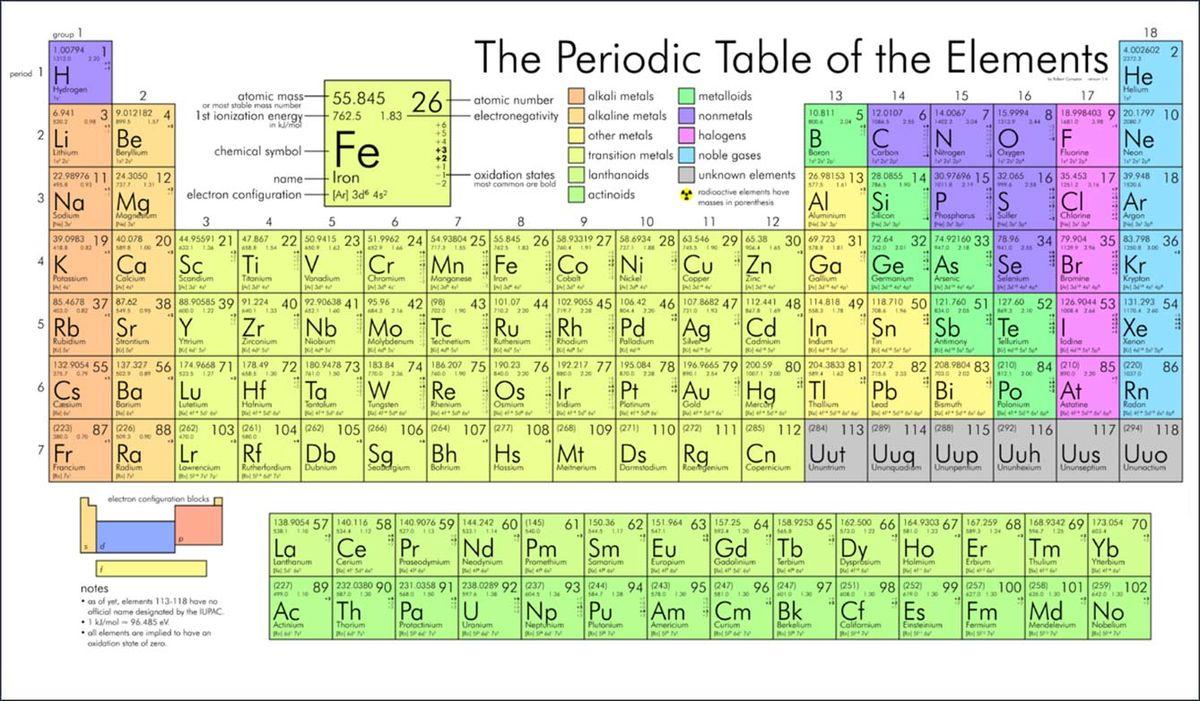
- Q1The elements in the periodic table are organized by....decreasing atomic numberincreasing atomic numberincreasing atomic massdecreasing atomic size120s
- Q2When 2 or more substances combine, but each keeps its own properties, the new combination is a (an)mixturepure substanceelementcompound120s
- Q3The atomic number of Magnesium (Mg) is 12. This means that its nucleus must contain12 neutrons and no protons12 protons and no electrons6 protons and 6 neutrons6 neutrons and 6 electrons120s
- Q4Which is an example of an atomThe smallest unit of NH3The smallest unit of NaOHThe smallest unit of H2OThe smallest unit of He120s
- Q5This image is an example of a model of a (an)compoundatommixturemolecule120s
- Q6This image is an example of a model of a (an)mixtureelementatomcompound120s
- Q7The outlined elements have a major characteristic in common: they are allmetalsnonmetalssolids at room temperaturemetalloids120s
- Q8Neutrons are found in the nucleus and separate the other particles so that the strong force can hold the nucleus together. Which particles are POSITIVELY charged and need to be separated so that they do not repel inside the nucleus?quarkselectronsneutronsprotons120s
- Q9In the diagram of an atom represented here, B is.....an electrona protona neutronthe nucleus120s
- Q10In the diagram of an atom represented here, C is.....a protona neutronthe nucleusan electron120s
- Q11Look at the segment of the periodic table here. What of the following elements have more chemical and physical properties in common?Ti, Zr and HfK, Ca and ScFr, Ra and AcCo, Mt and Ra120s
- Q12In the classification of matter, letter B representssolutionsmoleculescompoundselements120s
- Q13In the classification of matter, letter C representscompoundselementssolutionsmolecules120s
- Q14In the classification of matter, two examples of substances classified as A would bea Mixture of Salt and WaterAir ; SteelWater ; GlucoseCalcium ; Hydrogen120s
- Q15In the classification of matter, two examples of substances classified as B would beWater ; Glucosea Mixture of Salt and SandCalcium ; HydrogenAir ; Steel120s