Mixtures
Quiz by Mrs. Clarici
Feel free to use or edit a copy
includes Teacher and Student dashboards
Measure skillsfrom any curriculum
Measure skills
from any curriculum
Tag the questions with any skills you have. Your dashboard will track each student's mastery of each skill.
With a free account, teachers can
- edit the questions
- save a copy for later
- start a class game
- automatically assign follow-up activities based on students’ scores
- assign as homework
- share a link with colleagues
- print as a bubble sheet
42 questions
Show answers
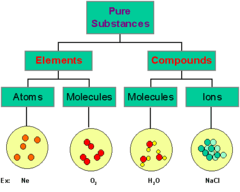
- Q1element or a compoundpure substancemixtureion30s
- Q2A combination of two or more substances that are not chemically combinedMixtureelementcompoundatom30s
- Q3poorly mixedheterogeneous mixturehomogeneous mixturesolution30s
- Q4A mixture in which particles can be seen and easily separated by settling or filtrationsupernateSuspensionprecipitatesolution30s
- Q5well mixed; same throughouthomogeneous mixtureheterogeneous mixturesuspension30s
- Q6A mixture that forms when one substance dissolves another; cannot see particlessolventSolutionsolute30s
- Q7A mixture that has only a little solute dissolved in a certain amount of solventdilute solutionsuper-saturated solutionsaturated solutionconcentrated solution30s
- Q8Substance that does the dissolving in a solution; more of itprecipitatesolutesolventsolution30s
- Q9technically dissolves everything;alcoholsolventsoluteuniversal solvent30s
- Q10unstable; higher saturation than saturated; crystallization occurs under right conditionsdilute solutionsaturated solutionsupersaturated solution30s
- Q11A substance that is dissolved in a solution; less of itsolventsolutionprecipitatesolute30s
- Q12A measure of how much solute can dissolve in a given solvent at a given temperaturesolubilitysolventsolute30s
- Q13A mixture that has a lot of solute dissolved in itprecipitatedilute solutionconcentrated solution30s
- Q14a solution that cannot dissolve any more solute under given conditionsunsaturated solutionsaturated solutiondilute solution30s
- Q15A solution that contains less solute; is able to dissolve additional solute under given conditionsunsaturated solutionsolutesolventsaturated solution30s