Periodic Table Quiz #2
Quiz by Derek Mauldin
High School
Chemistry (2017)
Texas Essential Knowledge and Skills (TEKS)
Feel free to use or edit a copy
includes Teacher and Student dashboards
Measures 2 skills from
Measures 2 skills from
With a free account, teachers can
- edit the questions
- save a copy for later
- start a class game
- automatically assign follow-up activities based on students’ scores
- assign as homework
- share a link with colleagues
- print as a bubble sheet
20 questions
Show answers
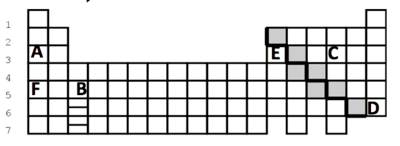
- Q1Which symbol in the picture represents an element that has a full octet (8) valence electrons?DABE60s112.35.c.5.b
- Q2Which element in the picture has the smallest atomic radius?FBCA60s112.35.c.5.b
- Q3Which 2 elements have the same number of valence electrons?A and DA and FF and BE and C60s112.35.c.5.b
- Q4Which element in the picture has 3 valance electrons?BDAE60s112.35.c.5.b
- Q5Which element in the picture is a member of a group that is made up of only gases?ACED60s112.35.c.5.b
- Q6Which elements are a member of the same period?F and DA, E and CA and FC and D60s112.35.c.5.b
- Q7Is the atom pictured a metal, nonmetal or metalloid?NonmetalCannot be determinedMetalMetalloid60s112.35.c.5.b
- Q8In which group does this atom with one valence electron belong?Alkaline Earth MetalsAlkali MetalsNoble GasesHalogens60s112.35.c.5.b
- Q9The element in period 3 with six valence electrons isCarbonSiliconSodiumSulfur60s112.35.c.5.b
- Q10Which of the following will have a larger atomic radius than Zinc , Zn?Strontium, SrGallium, GaMagnesium, MgAluminum, Al60s112.35.c.5.c
- Q11Which of the elements listed has the largest electronegativity and the highest ionization energy?Fluorine, FBromine, BrIodine, IChlorine, Cl60s112.35.c.5.c
- Q12The International Union of Pure and Applied Chemistry (IUPAC) have recently announced they have added four of the newest discovered elements, all with seven shells (rings) of electrons: Nihonium (Nh-113), Moscovium (Mc-115), Tennessine (Ts-117), Organessum (Og-118). In which area of the periodic table do all of these elements belong?A new period, Period 8Alkali MetalsNoble GasesPeriod 760s
- Q13Which group on the periodic table exhibits both a high ionization energy and a large electronegativity?HalogensTransition MetalsAlkaline Earth MetalsAlkali Metals60s112.35.c.5.c
- Q14Which of the following will have a lower ionization energy than Scandium, Sc?Oxygen, OCalcium, CaTitanium, TiHelium, He60s112.35.c.5.c
- Q15As the elements in group 15 are considered in order from bottom to top, the electronegativity of each successive element generallydecreasesstays the samenone of the aboveincreases60s112.35.c.5.c