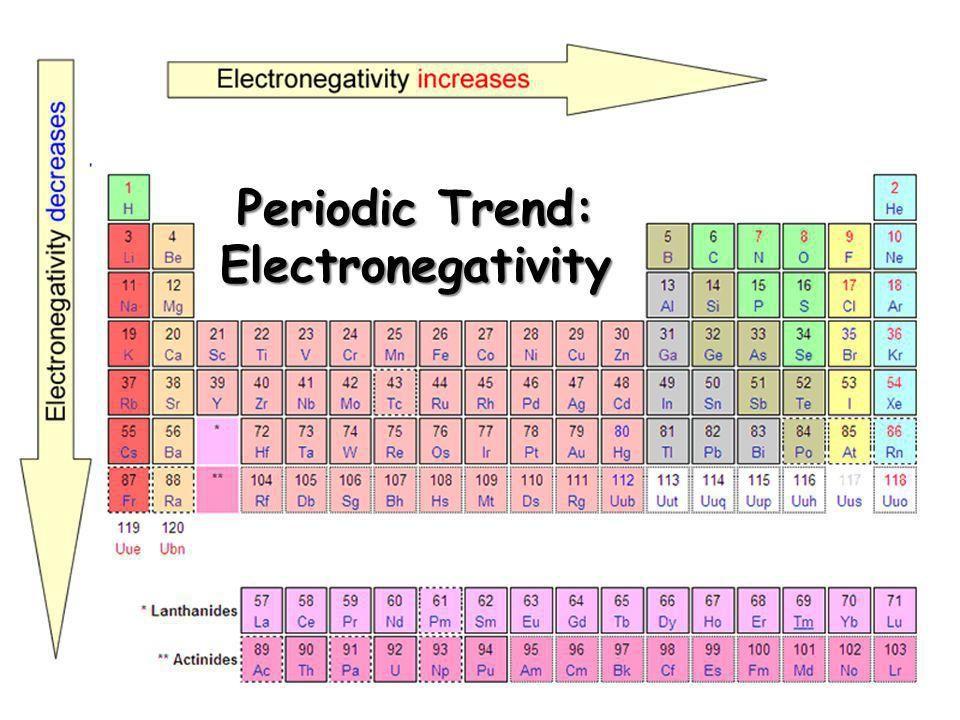
Periodic Trends
Quiz by Mr. Carousso
Feel free to use or edit a copy
includes Teacher and Student dashboards
Measure skillsfrom any curriculum
Measure skills
from any curriculum
Tag the questions with any skills you have. Your dashboard will track each student's mastery of each skill.
With a free account, teachers can
- edit the questions
- save a copy for later
- start a class game
- automatically assign follow-up activities based on students’ scores
- assign as homework
- share a link with colleagues
- print as a bubble sheet
20 questions
Show answers
- Q1Which of the following elements has the highest electronegativity?AtHFFr60s
- Q2Which of the following elements has the lowest electronegativity?FrHFAt60s
- Q3Which of the following elements has the highest electronegativity? (Column 1/1A = Alkali Metals)RbFrKLi60s
- Q4Which of the following elements has the highest electronegativity? (Period/Row 2)BLiBeC60s
- Q5Which element has the largest radius (biggest size)?AtHHeFr60s
- Q6Which element has the smallest radius (biggest size)?FAtHFr60s
- Q7Which element has the smallest radius (biggest size)? (Column 2A/2 = Alkali Earth Metals)BeMgRaBa60s
- Q8Which element has the largest radius (biggest size)? (Column 2A/2 = Alkali Earth Metals)BeRaMgBa60s
- Q9Which element has the largest radius (biggest size)? (Period/Row 3)KScTiCa60s
- Q10Which element has the smallest radius (biggest size)? (Period/Row 3)CaScTiK60s
- Q11Which of the following ions are cations?Na+Cl-F-Br-60s
- Q12Which of the following ions are cations?Li+F-Br-Cl-60s
- Q13Which of the following ions are anions?F-Li+Na+K+60s
- Q14Which of the following ions are anions?Br-Na+Li+K+60s
- Q15Which element has the largest Ionization energy?FAtHFr60s