Reading and Interpreting Heating Curves
Quiz by Amoi Salmon
Feel free to use or edit a copy
includes Teacher and Student dashboards
Measure skillsfrom any curriculum
Tag the questions with any skills you have. Your dashboard will track each student's mastery of each skill.
- edit the questions
- save a copy for later
- start a class game
- automatically assign follow-up activities based on students’ scores
- assign as homework
- share a link with colleagues
- print as a bubble sheet
- Q1
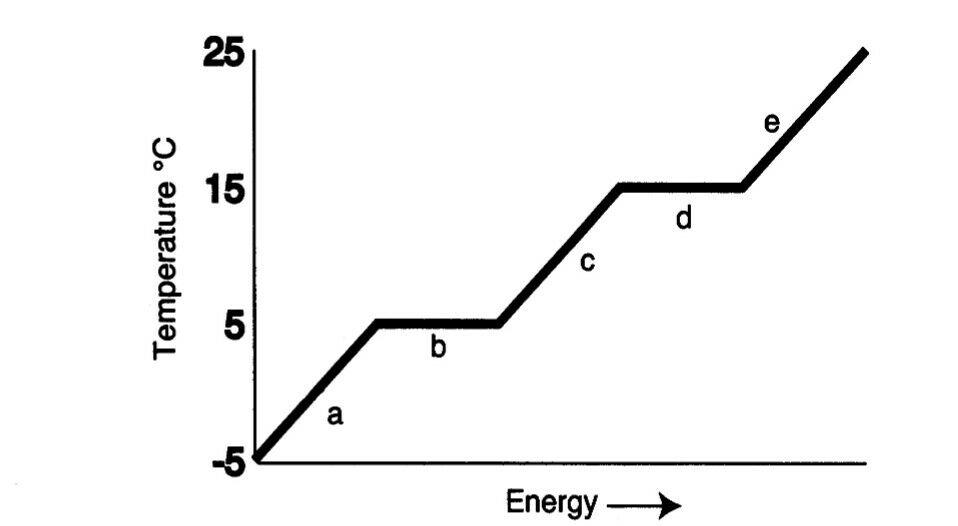
What is the freezing point of the substance?
25 oC
5 oC
15 oC
-5 oC
30s - Q2
What is the boiling point of the substance?
5 oC
-5 oC
25 oC
15 oC
30s - Q3
What is the melting point of the substance?
-5 oC
5 oC
25 oC
15 oC
30s - Q4
What letter represents the range where the solid is being warmed?
a
c
e
d
b
30s - Q5
What letter represents the range where the liquid is being warmed?
a
b
e
c
d
30s - Q6
What letter represents the range where the vapor is being warmed?
a
d
c
b
e
30s - Q7
What letter represents the melting of the solid?
a
c
b
d
e
30s - Q8
What represents the vaporization of the liquid?
d
c
e
b
a
30s - Q9
What letter(s) represents change in potential energy?
c only
e only
both b and d
b only
30s - Q10
What letter(s) show change in Kinetic Energy?
b and d only
a and c only
a, c, and e
e only
30s - Q11
What letter represents condensation?
a
c
e
d
b
30s - Q12
What letter represents crystallization?
a
b
c
e
d
30s - Q13
What areas of the graph show that the intermolecular forces of attraction are being broken between molecules?
d because only liquids have a strong intermolecular force
a and c only because the temperature is increasing
b and d only because they are flat and the temperature is not changing during a phase change
a because only solids have a strong intermolecular force
30s - Q14
HCl(s) ----> HCl(g) can be described as an...
Endothermic process
Exothermic Process
30s - Q15
HCl (g) ----> HCl (l) can be described as an
Endothermic process
Exothermic process
30s