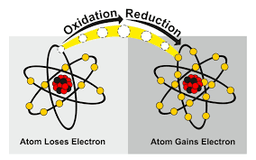
REDOX REACTIONS
Quiz by PREETI BIJU
Feel free to use or edit a copy
includes Teacher and Student dashboards
Measure skillsfrom any curriculum
Tag the questions with any skills you have. Your dashboard will track each student's mastery of each skill.
- edit the questions
- save a copy for later
- start a class game
- automatically assign follow-up activities based on students’ scores
- assign as homework
- share a link with colleagues
- print as a bubble sheet
- Q1
The key characteristic of redox reactions is the
noble gas electron configurations of each species
passing of one or more electrons from one species to another
fixed electron configurations of each species
none of the above
30s - Q2
_________ are assigned to each element as a formalism to keep track of the movement of electrons between species.
Molar masses
Physical states
Oxidation numbers
stoichiometric coefficients
30s - Q3
Simply stated, the gain of electrons is
sublimation
reduction
oxidation
hydration
30s - Q4
During oxidation, the oxidation number of a species becomes
zero
more neutral
more negative
more positive
30s - Q5
The goal of a redox reaction is to keep the number of electrons lost
equal to the number of electrons gained
less than the number of electrons gained
zero
more than the number of electrons gained
30s - Q6
Changes in the __________ can also be used to balance redox equations.
physical states
molecular structures
number of species
oxidation numbers
30s - Q7
What is the oxidation number of N in NO2-1?
-4
+3
-3
-1
30s