S3 Exp Science Chem Bridging 2024 #02
Quiz by Boh Peng
Feel free to use or edit a copy
includes Teacher and Student dashboards
Measure skillsfrom any curriculum
Tag the questions with any skills you have. Your dashboard will track each student's mastery of each skill.
- edit the questions
- save a copy for later
- start a class game
- automatically assign follow-up activities based on students’ scores
- assign as homework
- share a link with colleagues
- print as a bubble sheet
- Q1
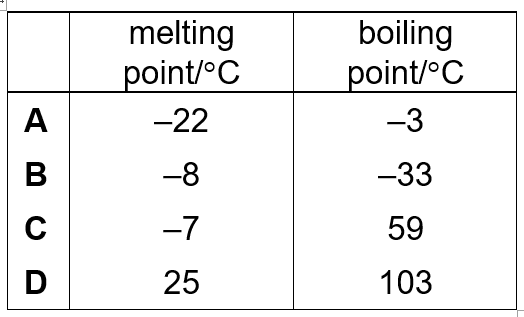
Bromine is a liquid at 20 °C.
What is the melting point and boiling point for bromine?
A
B
C
D
30s - Q2
An atom of potassium is represented as K.
Which row shows the number of protons, neutrons and electrons in the atom?
A
B
C
D
30s - Q3
Sodium chloride is an ionic compound.
Which statement about sodium chloride is not correct?
Sodium ions and chloride ions are oppositely charged.
Sodium chloride has a high melting point.
Sodium chloride solid conducts electricity.
Sodium chloride exists as a lattice.
30s - Q4
Iron(III) sulfate is composed of Fe3+ and SO42–ions.
Which values of x and y in Fex(SO4)ygive the correct formula of iron(III) sulfate?
A
B
C
D
30s - Q5
Which diagram shows the arrangement of the atoms in stainless steel?
30s - Q630s
- Q7
Four elements have the following electronic configurations.
W 2.8.1
X 2.8.2
Y 2.8.5
Z 2.8.8
Which statement is correct?
All four elements are in the same group of the Periodic Table.
All four elements are in the same period of the Periodic Table.
W is a metal but X, Y and Z are non-metals.
X is a metal but W, Y and Z are non-metals.
30s - Q8
The diagrams represent different arrangement of atoms.
Which diagram represents an alloy?
30s - Q9
The nucleon number of an isotope of bromine is 81.
How many protons, neutrons and electrons are present in an atom of this isotope?
A
B
C
D
30s - Q10
Why does molten sodium chloride conduct electricity?
An electron is completely transferred from sodium to chlorine.
Electrons in sodium chloride are free to move.
Sodium ions are strongly attracted to the chloride ions.
The sodium ions and the chloride ions are free to move.
30s - Q11
An element X forms an ion X3–.
Which group of the Periodic Table is this element found in?
Group 1
Group 13
Group 15
Group 17
30s - Q12
Which substance represents a metal?
A
B
C
D
30s - Q13
The diagrams show the electronic structures of four elements.
Which elements are metals?
1and 2
1and 3
2and 4
3and 4
30s - Q14
The particles in substance Zare widely spaced and able to move freely.
Z changes to state in which the particles are in contact but still able to move freely.
What is this change called?
condensation
evaporation
freezing
melting
30s - Q15
The nucleon number and proton number of an atom of X and an atom of Y are shown.
Which statement about X and Y is correct?
An atom of X has fewer electrons than an atom of Y.
An atom of X has fewer neutrons than an atom of Y.
X is above Y in the same group of the Periodic Table.
X is in the same period in the Periodic Table as Y.
30s