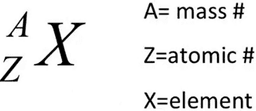
SHORTHAND NOTATION OF A NEUTRAL ATOM
Quiz by Kim Dela Cruz
Feel free to use or edit a copy
includes Teacher and Student dashboards
Measure skillsfrom any curriculum
Tag the questions with any skills you have. Your dashboard will track each student's mastery of each skill.
- edit the questions
- save a copy for later
- start a class game
- automatically assign follow-up activities based on students’ scores
- assign as homework
- share a link with colleagues
- print as a bubble sheet
- Q1
Which phrase describes the different isotopes of an element?
B. Same number of protons and a different number of electrons.
A. Same number of electrons and a different number of protons.
C. Same number of protons and a different number of neutrons.
D. Same number of neutrons and a different number of protons.
60s - Q2
Which ion has the greatest mass?
A. A
D. Z
B. E
C. G
30s - Q3
Which statement describes two different isotopes of carbon?
B. The isotopes contain the same number of neutrons but have a different atomic number.
C. The isotopes contain a different number of neutrons but have the same atomic number.
D. The isotopes contain a different number of neutrons and have a different atomic number.
A. The isotopes contain the same number of neutrons and have the same atomic number.
30s - Q4
Which element is listed with the number of protons in each of its atoms?
C. oxygen, 16
D. phosphorus, 16
B. silicon, 14
A. nitrogen, 14
30s - Q5
A potassium atom has a mass number of 37. What is the number of neutrons in this atom?
C. 22
D. 37
B. 18
A. 15
30s - Q6
Which phrase describes the different isotopes of an element?
D. Same number of neutrons and a different number of protons.
C. Same number of protons and a different number of neutrons.
A. Same number of electrons and a different number of protons.
B. Same number of protons and a different number of electrons.
60s