Solutions and Solubility Review
Quiz by Trina Boyle
Feel free to use or edit a copy
includes Teacher and Student dashboards
Measure skillsfrom any curriculum
Measure skills
from any curriculum
Tag the questions with any skills you have. Your dashboard will track each student's mastery of each skill.
With a free account, teachers can
- edit the questions
- save a copy for later
- start a class game
- automatically assign follow-up activities based on students’ scores
- assign as homework
- share a link with colleagues
- print as a bubble sheet
22 questions
Show answers
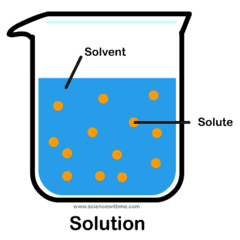
- Q1evenly mixed substance, containing solvent and solutesolutionscolliodsubstance30s
- Q2larger amount, dissolves solutesolutesolvent30s
- Q3smaller amount, is dissolved in solventsolute30s
- Q4measures the motion or kinetic energy of particlestemperature30s
- Q5The amount of force exerted per unit area of a surfacetemperaturepressure30s
- Q6the amount of a solute that dissolves in a specific amount of solution at a specific temperaturesolubilitydissolvablitymeasurability30s
- Q7graph showing the solubility of certain substancessolubility measurementsolubility curvesolubility slope30s
- Q8the total amount of exposed surface of a substanceareasurface volumesurface area30s
- Q9wateroften called "universal solvent"30s
- Q10aqueous solutiontoluenewateralcohol30s
- Q11molecules spread far apartgassolidliquid30s
- Q12molecules next to each other, but not fixedsolidliquidgas30s
- Q13molecules in a fixed positiongasliquidsolid30s
- Q14decrease in temperature (usually when solute is a solid)speeds up solubilityslows down solubility30s
- Q15increase in temperature (usually when solute is a solid)decrease rate of solubilityincreases rate of solubility45s