(TEKS 8.5 A-E) STAAR Review
Quiz by Jennifer Bradley
Grade 8
Science (2010) (Archived)
Texas Essential Knowledge and Skills (TEKS)
Feel free to use or edit a copy
includes Teacher and Student dashboards
Measure skillsfrom any curriculum
Measure skills
from any curriculum
Tag the questions with any skills you have. Your dashboard will track each student's mastery of each skill.
With a free account, teachers can
- edit the questions
- save a copy for later
- start a class game
- automatically assign follow-up activities based on students’ scores
- assign as homework
- share a link with colleagues
- print as a bubble sheet
8 questions
Show answers
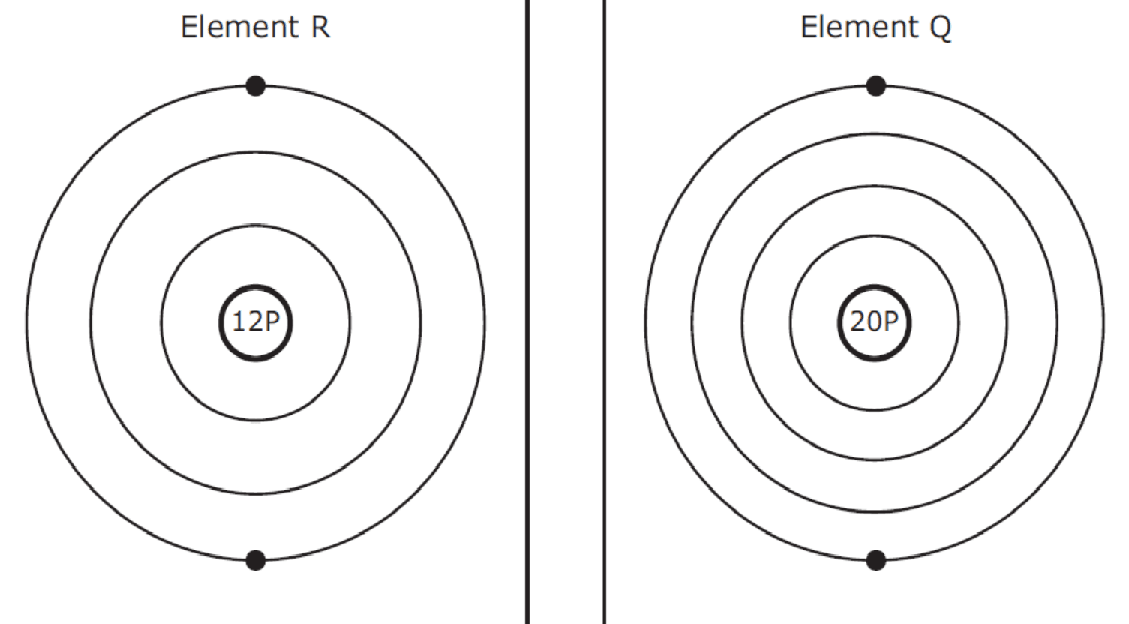
- Q1Element R and Element Q have the same number of valence electrons. These elements both have similar chemical behavior, but Element R has fewer energy levels than Element Q. Which statement best describes the positions of the two elements in the periodic table?The two elements are in the same group, with Element R just above Element Q.The two elements are in the same group, with Element Q at the top of the group and Element R at the bottom.The two elements are side by side in the same period, with Element Q to the left of Element R.The two elements are in the same period, with Element R the first element in the period and Element Q the last element.45s
- Q2Some students are using samples of different substances for a lab investigation. They plan to observe the physical properties of each substance and record their observations in a table like the one below. Which of the substances observed are elements?NO,Fe,Sr,andAlS, Fe, O, Sr, and AlNO, O,andLiFS, O, and LiF45s
- Q3A student obtains two strips of magnesium, Mg, ribbon that are each 3 cm long. One strip of magnesium is placed in a test tube containing 5 mL of water, and the other strip is placed in a test tube containing 5 mL of hydrochloric acid, HCl. Both liquids are at room temperature. The student’s observations are recorded in the table. Which statement is not supported by the student’s observations?Energy is released in the reaction involving hydrochloric acid.A chemical reaction takes place between magnesium and hydrochloric acid.A gas is released in Test Tube 2.The substances in both test tubes are reactive only at high temperatures.45s
- Q445s
- Q5Four students were given a list of compounds and asked to identify which ones are organic. Which student correctly identified the organic compounds in the list?Student NStudent LStudent MStudent K45s
- Q6Four students were asked to name the parts of an atom that determine the atom’s identity and chemical properties. The students’ responses are shown in the table below. Which student’s responses are correct?Student 2Student 1Student 4Student 345s
- Q7Strontium phosphate, Sr3(PO4)2 is a crystalline substance used in medicine and industry. How many phosphorus atoms are represented in the formula for Sr 3(PO4)2?438245s
- Q8A chemical reaction in which calcium carbonate, CaCO3, is decomposed results in the production of two simpler compounds. What mass of calcium carbonate, to the nearest hundredth of a gram, is decomposed in this reaction?49.976.0321.9728.0045s