Topic 3 Lesson 2 Test
Quiz by Heather Reeve
Feel free to use or edit a copy
includes Teacher and Student dashboards
Measure skillsfrom any curriculum
Measure skills
from any curriculum
Tag the questions with any skills you have. Your dashboard will track each student's mastery of each skill.
With a free account, teachers can
- edit the questions
- save a copy for later
- start a class game
- automatically assign follow-up activities based on students’ scores
- assign as homework
- share a link with colleagues
- print as a bubble sheet
13 questions
Show answers
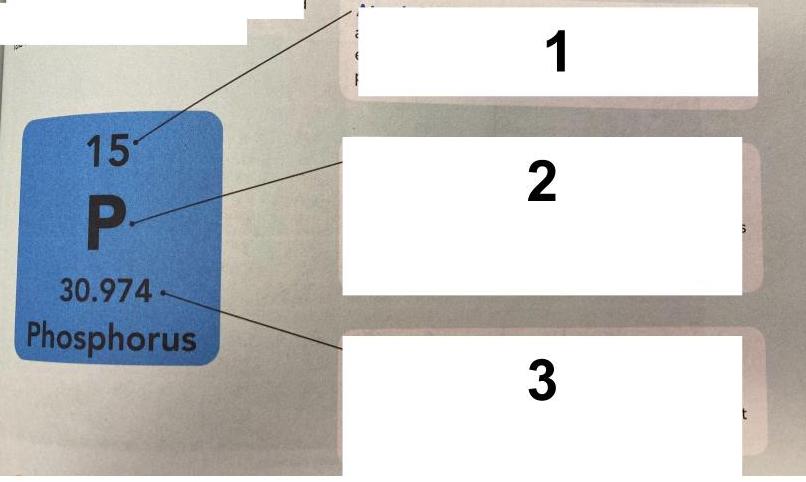
- Q1This is from the APE MAN worksheet and the Topic 3 Lesson 2 Nearpod: What does #1 in the picture represent?chemical numberatomic massatomic numberchemical symbol30s
- Q2This is from the APE MAN worksheet and the Topic 3 Lesson 2 Nearpod: What does #2 in the picture represent?atomic numberchemical symbolchemical numberatomic mass30s
- Q3This is from the APE MAN worksheet and the Topic 3 Lesson 2 Nearpod: What does #3 in the picture represent?chemical symbolatomic numberchemical numberatomic mass30s
- Q4Gold, Sliver, and Platinum are are highly valuable metals because of their similar chemical properties, such as reactivity, high ductility, and high luster. Which of the following is another example of a precious metal, based on its location on the Periodic Table? Use the periodic table on textbook pp. 526-527 if you need a better look.palladiumnitrogenzirconiumoxygen30s
- Q5Neon lights are commonly used in signs because they emit attractive colors of lights. Orange neon lights are filled with neon gas. Different gases produce different colors. Which of these elements would you least expect to find in a neon light, based on its position on the Periodic Table? Use the periodic table on textbook pp. 526-527 if you need a better look.kryptonargonxenonchlorine30s
- Q6Jailah needs a replacement for lithium for a science experiment. She tries silicon, but it does not work. Using what you know about patterns in the periodic table, which advice would you give Jailah? Use the periodic table on textbook pp. 526-527 if you need a better look.Try an element in the same group as lithium.Try an element in the same period as lithium.Try a non-metal from the halogen group.Try a lanthanide series element.30s
- Q7What element is found in Group 6 and Period 4? Use the periodic table on textbook pp. 526-527 if you need a better look.MolybdenumTungstenSeaborgiumChromium30s
- Q8Which element is Chromium more similar to? Use the periodic table on textbook pp. 526-527 if you need a better look.TungstenThalliumLithiumiron30s
- Q9This is from our Topic 3 Lesson 2 Notes: How are the atoms arranged in the modern periodic table?by decreasing atomic numberby increasing atomic numberby increasing atomic massby decreasing atomic mass30s
- Q10This is from our Topic 3 Lesson 2 Notes: Who organized elements into the first periodic table?RutherfordDaltonMendeleevThomson30s
- Q11This is from our Topic 3 Lesson 2 Notes and your brain: A museum wants to store their valuable documents in cases that contain noble gases that will protect the documents. She should not choose one of the other gases because they are too _________.smellyexpensivereactiveunreactive30s
- Q12This is from the APE MAN worksheet: From the information in this picture, what is the most likely number of neutrons in an atom of titanium?264825.9047.9030s
- Q13This is from our Topic 3 Lesson 2 Vocabulary: Atomic Mass: Assume that one particular tom of rhodium (Rh) has a mass of 102. Why does this number not exactly match the atomic mass of 102.91 listed on the periodic table for this element?The atomic mass is an average of all the isotopes of that element.The atomic mass only includes the average of all the neutrons.The atomic mass only includes the average of all the electrons.The atomic mass only includes the average of all the protons.30s