Topic: 8.5 Matter and Energy (2)
Quiz by Grade 8 Science - Texas Education Agency
Grade 8
Science (2017)
Texas Essential Knowledge and Skills (TEKS)
Feel free to use or edit a copy
includes Teacher and Student dashboards
Measures 5 skills from
Measures 5 skills from
With a free account, teachers can
- edit the questions
- save a copy for later
- start a class game
- automatically assign follow-up activities based on students’ scores
- assign as homework
- share a link with colleagues
- print as a bubble sheet
21 questions
Show answers
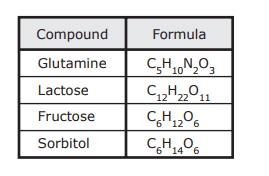
- Q1The table lists some compounds found in foods and their formulas. Based on this information, which of these statements is NOT true?A molecule of lactose contains twice as many carbon atoms as a molecule of sorbitol does.A molecule of fructose contains four more atoms than a molecule of glutamine does.A molecule of lactose contains twice as many atoms as a molecule of fructose does.A molecule of sorbitol contains three more oxygen atoms than a molecule of glutamine does.60s8.5d
- Q2A chemist made the table below to record some atomic properties of four elements. Based on the information in the table, which conclusion about the chemical reactivity of these elements is valid?Gallium is the most reactive because it has many more neutrons than protons.Fluorine is the most reactive because it has 7 electrons in the outer shell.Boron is the most reactive because it has the most protons.Silicon is the most reactive because it has an equal number of protons and neutrons.60s8.5b
- Q3Which pair of properties describes the elements in Group 18?They are gaseous at room temperature and chemically stable.They are magnetic and boil at low temperatures.They have eight valence electrons and are flammable.They are chemically stable and liquid at room temperature.60s8.5c
- Q4What is the total number of protons, neutrons, and electrons in a cadmium, Cd, atom that has a mass number of 112? Be sure to use the correct place value.Users enter free textType an Answer60s8.5a
- Q560s8.5d
- Q6A student is studying calcium, a highly reactive element that humans need for strong bones. Which characteristic of calcium is most closely related to its chemical reactivity?The 20 protons in each atom of calciumThe atomic mass of calcium, which is 40.078 amuThe density of calcium, which is 1 5.4 g/cm$^3$The 2 valence electrons in each atom of calcium60s8.5b
- Q7A model of a beryllium atom is shown below. What types of particles are found in the cloud surrounding the atom’s nucleus?Neutral particles and positively charged particlesNegatively charged particles onlyPositively charged particles onlyPositively charged particles and negatively charged particles60s8.5a
- Q8The chemical formula for sodium sulfate is $Na_{2}SO_{4}$ . How many sulfur atoms are in the formula for sodium sulfate?672160s8.5d
- Q9What is the mass number of a potassium (K) atom that has 20 neutrons?1918392060s8.5a
- Q10Users enter free textType an Answer60s8.5d
- Q11A student is studying the ways different elements are similar to one another. Diagrams of atoms from four different elements are shown below. Which two atoms are of elements in the same group in the periodic table?Atom 2 and Atom 3Atom 1 and Atom 2Atom 3 and Atom 4Atom 1 and Atom 460s8.5c
- Q12The table below lists three characteristics of an atom of an element. An atom of which element is described by the data in the table?Rubidium (Rb)Astatine (At)Radon (Rn)Cadmium (Cd)60s8.5b
- Q13Element R and Element Q have the same number of valence electrons. These elements both have similar chemical behavior, but Element R has fewer energy levels than Element Q. Which statement best describes the positions of the two elements in the periodic table?The two elements are in the same period, with Element R the first element in the period and Element Q the last element.The two elements are side by side in the same period, with Element Q to the left of Element RThe two elements are in the same group, with Element R just above Element Q.The two elements are in the same group, with Element Q at the top of the group and Element R at the bottom.60s8.5c
- Q14What is the difference between the number of electrons in an atom of selenium, Se, and the number of electrons in an atom of aluminum, Al? Be sure to use the correct place value.Users enter free textType an Answer60s8.5a
- Q15Four students were asked to name the parts of an atom that determine the atom’s identity and chemical properties. The students’ responses are shown in the table below. Which student’s responses are correct?Student 4Student 2Student 1Student 360s8.5b