Periodic Table Section 2 Review (Types of Elements)
Quiz by Aaron Holley
Feel free to use or edit a copy
includes Teacher and Student dashboards
Measure skillsfrom any curriculum
Tag the questions with any skills you have. Your dashboard will track each student's mastery of each skill.
- edit the questions
- save a copy for later
- start a class game
- automatically assign follow-up activities based on students’ scores
- assign as homework
- share a link with colleagues
- print as a bubble sheet
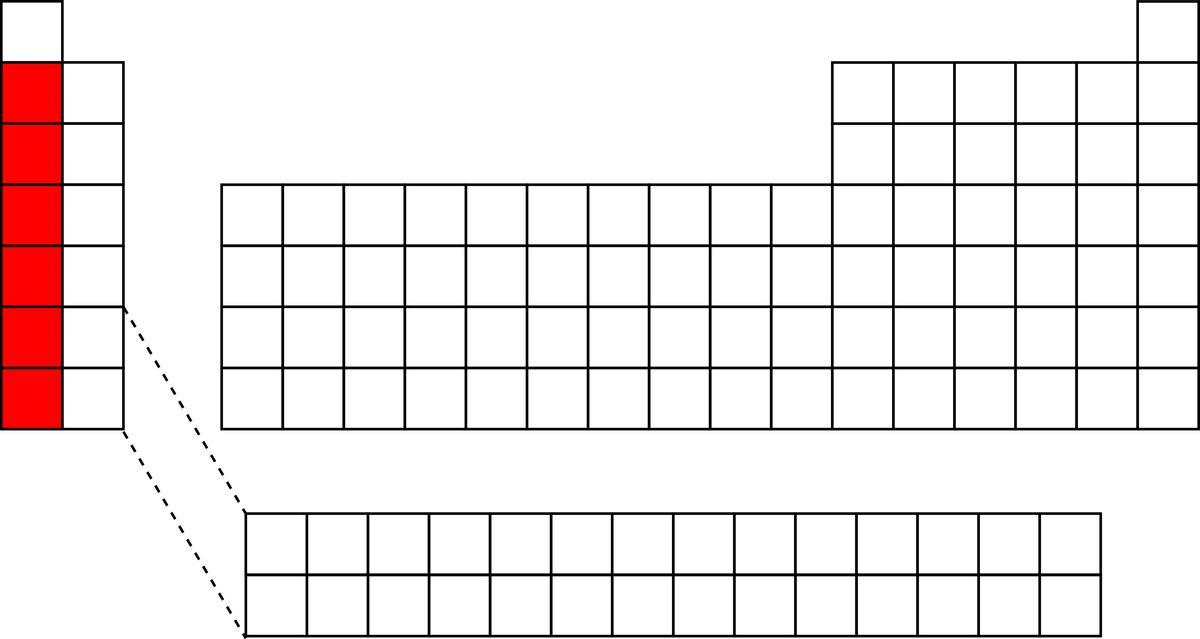
- Q1
The highlighted elements shown below represent what type of elements?
actinides
alkaline earth metals
halogens
noble gases
alkali metals
transition metals
60s - Q2
The highlighted elements shown below represent what type of elements?
halogens
transition metals
alkaline earth metals
actinides
noble gases
alkali metals
60s - Q3
The highlighted elements shown below represent what type of elements?
actinides
halogens
noble gases
alkali metals
alkaline earth metals
transition metals
60s - Q4
The highlighted elements shown below represent what type of elements?
post-transition metals
transition metals
halogens
inner transition metals
noble gases
lanthanides
60s - Q5
The highlighted elements shown below represent what type of elements?
halogens
lanthanides
post-transition metals
inner transition metals
transition metals
noble gases
60s - Q6
The highlighted elements shown below represent what type of elements?
lanthanides
inner transition metals
noble gases
halogens
transition metals
post-transition metals
60s - Q7
The highlighted elements shown below represent what type of elements?
lanthanides
halogens
noble gases
inner transition metals
post-transition metals
transition metals
60s - Q8
The highlighted elements shown below represent what type of elements?
inner transition metals
post-transition metals
halogens
transition metals
lanthanides
noble gases
60s - Q9
The highlighted elements shown below represent what type of elements?
lanthanides
halogens
noble gases
inner transition metals
transition metals
metalloids
60s - Q10
The highlighted elements shown below represent what type of elements?
lanthanides
metalloids
halogens
transition metals
noble gases
inner transition metals
60s - Q11
Elements in groups 1A through 8A are referred to as ____.
representative elements
transition metals
archetypal atoms
emblematic elements
60s - Q12
The electrons in the highest occupied energy level are called ____.
energetic electrons
inner shell electrons
outer shell electrons
valence electrons
60s - Q13
The electrons that are NOT in the highest occupied energy level are called ____.
energetic electrons
valence electrons
outer shell electrons
inner shell electrons
60s - Q14
Which of the following affects the properties of the elements?
atomic number
mass number
inner shell electrons
valence electrons
30s - Q15
Which of the following affects the properties of the elements?
the number of valence electrons
both the number of valence electrons AND the subshell where the valence electrons are found
the number of protons in the nucleus
the subshell where valence electrons are found
30s