Unit 1: Atoms Basics Prior Knowledge Check
Quiz by Stephen Spitler
Feel free to use or edit a copy
includes Teacher and Student dashboards
Measure skillsfrom any curriculum
Tag the questions with any skills you have. Your dashboard will track each student's mastery of each skill.
- edit the questions
- save a copy for later
- start a class game
- automatically assign follow-up activities based on students’ scores
- assign as homework
- share a link with colleagues
- print as a bubble sheet
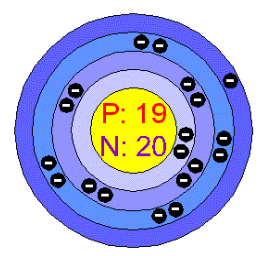
- Q1
What is the atomic number of this element?
39
I don't know
19
20
60s - Q2
What is the name of the element containing fourteen (14) protons?
I don't know
Carbon
Silicon
Nitrogen
120s - Q3
What is the mass number of the element shown in the image?
35
29
I don't know
64
120s - Q4
What particle(s) can be found in the nucleus of an atom?
Protons AND neutrons
Protons AND electrons
ONLY protons
I don't know
60s - Q5
Select the option that has the correct subatomic particle and charge from the options below.
I don't know
protons=positive... neutrons=neutral
protons=positive... neutrons=negative
60s - Q6
Which of the following is the correct isotope name for an element that has 20 protons and 24 neutrons?
Calcium-24
I don't know
Calcium-44
Calcium
60s - Q7
Which of the following images represents a hydrogen isotope with 2 neutrons?
I don't know
60s - Q8
Based on the element tile shown in the image, what is the most common isotope of phosphorus?
Phosophorus-31
I don't know
Phosophorus-30
Phosophorus-15
60s - Q9
In a science class, daily assignments count for 70% of your grade and tests count for 30%. If your overall daily assignment grade is 90 and your test average is 80, what score would be closest to your overall grade?
90
83
88
85
I don't know
120s - Q10
Select the face that best identifies your confidence in today's practice assessment.
45s