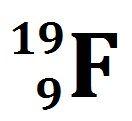
Unit 1: Isotopes & Percent Abundance
Quiz by Hugh King
Feel free to use or edit a copy
includes Teacher and Student dashboards
Measure skillsfrom any curriculum
Measure skills
from any curriculum
Tag the questions with any skills you have. Your dashboard will track each student's mastery of each skill.
With a free account, teachers can
- edit the questions
- save a copy for later
- start a class game
- automatically assign follow-up activities based on students’ scores
- assign as homework
- share a link with colleagues
- print as a bubble sheet
25 questions
Show answers
- Q1An isotope on the same element has a different number of _____.protonselectronsneutrons30s
- Q2What does the decimal number under the symbol of an element of the periodic table tell you?Atomic numberMass numberAtomic Mass
Number of croutons
30s - Q3To get the mass number you should add the _____ .protons and electrons
electrons and neutrons
proton and croutonsprotons and neutrons30s - Q4An atom of an element is neutral because the number of _____ are the same.protons and neutronsneutrons and electrons
neutrons and croutons
protons and electrons30s - Q5What subatomic particle gives an element its identity?Number of electronsNumber of protonsNumber of neutrons30s
- Q6How do you determine the mass number of an element?protons + neutronselectrons + neutronsprotons + electrons30s
- Q7If Magnesium-24 has 12 protons, how many protons does Magensium-25 have?25241230s
- Q8How many neutrons does Chromium-52 have?24285230s
- Q9How many neutrons does Chromium-50 have?24
50
265230s - Q10What is the mass number of Carbon-14?681430s
- Q11What is the mass number of Carbon-13?6
14
81330s - Q12How many protons does Beryllium-9 have?4
7
9530s - Q13How many protons does Beryllium-7 have?4
7
9530s - Q14What subatomic particle is used to identify an element?Number of protonsNumber of neutrons
Number of croutons
Number of electrons30s - Q15
Using the periodic table, what is the most abundant isotope of Lithium?
Lithium-7
Lithium-6
Lithium-6.94
Lithium-3
30s