Unit 3 Biological Carbon Cycle - Carbon Basics
Quiz by 7science PMS
Feel free to use or edit a copy
includes Teacher and Student dashboards
Measure skillsfrom any curriculum
Tag the questions with any skills you have. Your dashboard will track each student's mastery of each skill.
- edit the questions
- save a copy for later
- start a class game
- automatically assign follow-up activities based on students’ scores
- assign as homework
- share a link with colleagues
- print as a bubble sheet
- Q1
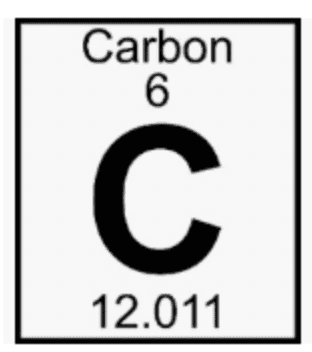
What is the atomic mass of the element in the square?
6
18
12.01
60s - Q2
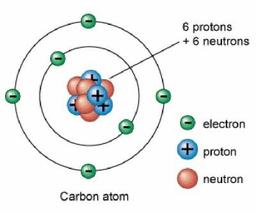
Using the element provided, determine the number of electrons in Carbon:
12.01
6
18
60s - Q3
What is the atomic number of the element in the square?
12.01
18
6
60s - Q4
Using the element provided, determine the number of protons in Carbon:
12.01
18
6
60s - Q5
What are the major elements of life?
Carbon, Hydrogen, Neon Oxygen, Potassium
Helium, Carbon, Calcium, Nitrogen, Oxygen
Hydrogen, Nitrogen, Calcium, Oxygen, Phosphorus
Nitrogen, Carbon, Phosphorus, Hydrogen, Oxygen
60s - Q6
What properties of carbon explain carbon’s ability to form different large and complex structures?
Carbon has 4 valence electrons which enable it to form ionic bonds with other elements and carbon can bond to other elements to form a large variety of complex structures.
Carbon has 2 valence electrons which enable it to form covalent bonds with other elements and carbon can bond to itself to form a small variety of complex structures.
Carbon has 4 sets of valence electrons which enable it to form covalent bonds with other elements and carbon can bond to itself to form a large variety of complex structures.
Carbon has 4 valence electrons which enable it to form covalent bonds with other elements and carbon can bond to itself to form a large variety of complex structures.
120s - Q7
What are the functions of the four macromolecules?
Users link answersLinking300s - Q8
Franky Frank is going to run a marathon race on Saturday. What would be the best thing for Franky Frank to eat to give him energy before the race?
carbohydrate, pasta
protein, steak
lipid, butter
carbohydrate, cookies
60s - Q9
Large molecules that form when smaller molecules join
micromolecules
atoms
elements
macromolecules
60s - Q10
Carbon is
the simples molecules of life.
the basic element that is part of all organic compounds.
the basic element of all molecules
60s - Q11
What is the main capacity of Carbon?
To make short compound molecules.
To make stable bonds with other carbon atoms .
To make unstable bonds with other carbon atoms.
60s - Q12
How many valence electrons does carbon have?
6
4
2
12
60s - Q13
How many bonds can carbon form?
2
12
6
4
60s - Q14
How do carbon atoms form many organic compounds?
By sharing their electrons with other metal and non-metal elements
By attracting other elements toward themselves to form bond
By mainly forming bonds with other carbon atoms and other elements
By transferring their electrons to the atoms of surrounding elements
60s